Aluminum Reacts With Oxygen to Produce Aluminum Oxide Chemical Equation
Aluminum reacts with oxygen according to the following reaction. Aluminium oxide also known as aluminum oxide is a chemical compound made from aluminium and oxygen.

Solved Aluminum Reacts With Oxygen To Produce Aluminum Oxide Chegg Com
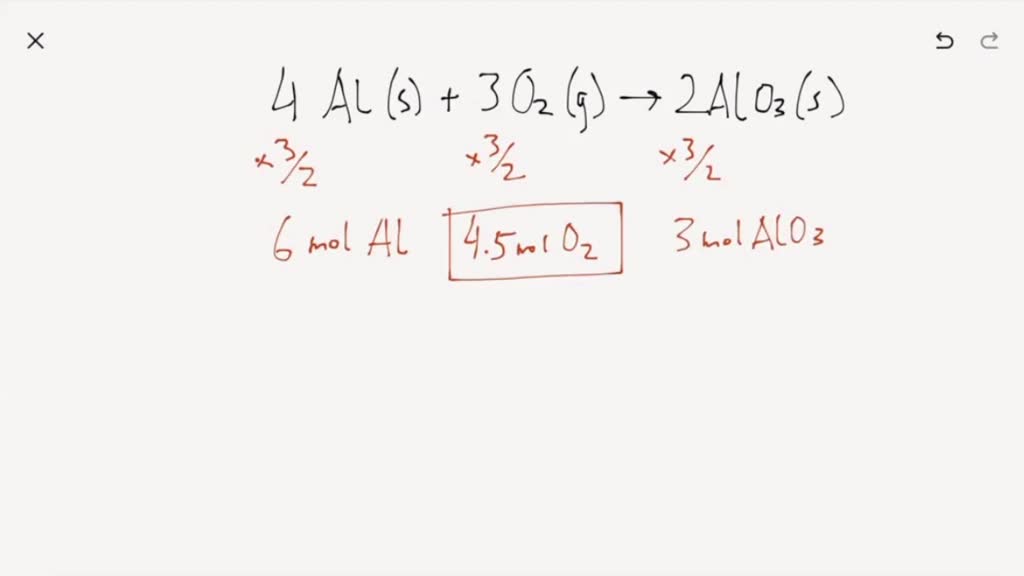
How many moles of oxygen are needed to react completely with six moles of aluminum.
. Thus when aluminum reacts with oxygen gas it produces aluminium oxide and the following chemical reaction is given by. It is therefore considered that aluminum does not react with air. Correct answer to the question 1 Aluminum reacts with oxygen to produce aluminum oxide what is the chemical equation.
So aluminum plus some amount of oxygen yields some amount of aluminum oxide. Question 11 A strip of aluminum metal Al reacts with oxygen gas O2 to produce aluminum oxide. A little number reacts with oxygen to form from oxide.
4Al 3O 2 2Al 2O 3 a. 4Al 3O 2 2Al 2 O 3. Were given that aluminium A l can react with oxygen gas O 2 to produce aluminium oxide A l 2 O 3.
The solubility of the substances. Aluminum can burn in oxygen dazzling white flame to form aluminum oxide Al2O3. Ferric oxide reacts with aluminium to produce aluminium oxide and iron.
Aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorbent desiccant or catalyst for organic reactions. Well we can see here that we lets just multiply. Will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation.
Aluminum reacts with oxygen to produce aluminum oxide according to the following equation. 4Al 3O2 2Al2O3. So that way theres four aluminum and six oxygen.
If 1 mol of hydrogen gas and 1 mol of oxygen gas are placed in a container and. So 302 Okay so we have our balanced. So we have here.
I really really hope that was helpful. Aluminum Al - 27 gmol. A little number reacts with oxygen to form from oxide.
Thermodynamic properties of substances. 4Al 3O2 -- 2Al2O3. This reaction is also known as synthesis reaction since there are two reactants which are aluminium and oxygen gas which on combining forms one product which is aluminium oxide.
O 263 mol O 117 mol O. A13 Question 12 What mass of ammonia is formed when 618 g of nitrogen gas reacts with excess hydrogen gas. Chemistry questions and answers.
Check the balance Aluminum react with oxygen to produce aluminum oxide. Write the balanced chemical equation for this reaction. F e 2 O 3 2 A l A l 2 O 3 2 F e.
4Al 3O2 2Al2O3 a. Aluminum reacts with oxygen to produce aluminum oxide as follows. How many grams of Al.
4Al s 3O2 g 2Al2O3 s A mixture of 8249 g of aluminum Picture 2698 gmol and 11765 g of oxygen Picture 3200 gmol is allowed to react. The chemical reaction of Aluminum oxygen yields aluminum oxide is in option d - 4Al 3O₂ 2Al₂O₃. The balanced chemical equation for this reaction is 4 A l s 3 O 2 g 2 A l 2 O 3 s.
Aluminium oxide is a chemical compound of aluminium and oxygen with the chemical formula Al 2 O 3It is the most commonly occurring of several aluminium oxides and specifically identified as aluminiumIII oxideIt is commonly called alumina and may also be called aloxide aloxite or alundum. The chemical equation when aluminum reacts with oxygen is. But this by two.
4Al 3O 2 2Al 2 O 3. Aluminum metal reacts with oxygen gas to produce aluminum oxide solid. If this oxide layer is damaged or removed the fresh surface of aluminum reacts with oxygen in the air.
Al O2 Al2O3 Balanced Chemical Reaction Details aluminium oxygen aluminium oxide Temperature. 4 Al s 3 0z g 2 AlOg s What is the maximum number of moles of AlO3 produced when 200 mol of Al is fully reacted with 175 mol of O2. The balanced chemical equation for the given reaction is.
How many grams of O 2 are needed to produce 5 moles of Al 2O 3. Reaction of aluminum with oxygen. Write down the balanced chemical equation.
Write the balanced chemical equation for this process. __N2g __ H29 _NH3g N1401. Therefore the balanced chemical equation for this reaction would be 2 Al 3 O --.
4 A l 3 O 2 2 A l 2 O 3. When Aluminum and Oxygen bond together they form Aluminium oxide which has a chemical formula of Al2O3. So first we gotta make a balanced equation.
How many grams of aluminum oxide is produced when 234 g of aluminum reacts with 342 g of oxygen in this balanced equation. What is the reducing agent in the redox reaction.

Solved Aluminum Reacts With Oxygen To Give Aluminum Oxide 4 Mathrm Al Mathrm S 3 Mathrm O 2 Mathrm G Longrightarrow 2 Mathrm Al 2 Mathrm O 3 Mathrm S What Amount Of Mathrm O 2 In Moles Is Needed For Complete Reaction With

Type Of Reaction For Al O2 Al2o3 Youtube
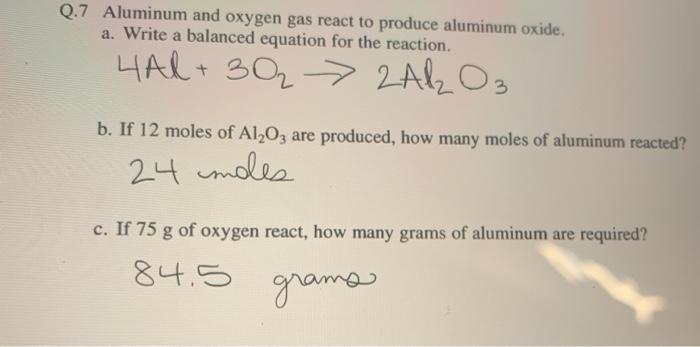
Solved Q 7 Aluminum And Oxygen Gas React To Produce Aluminum Chegg Com
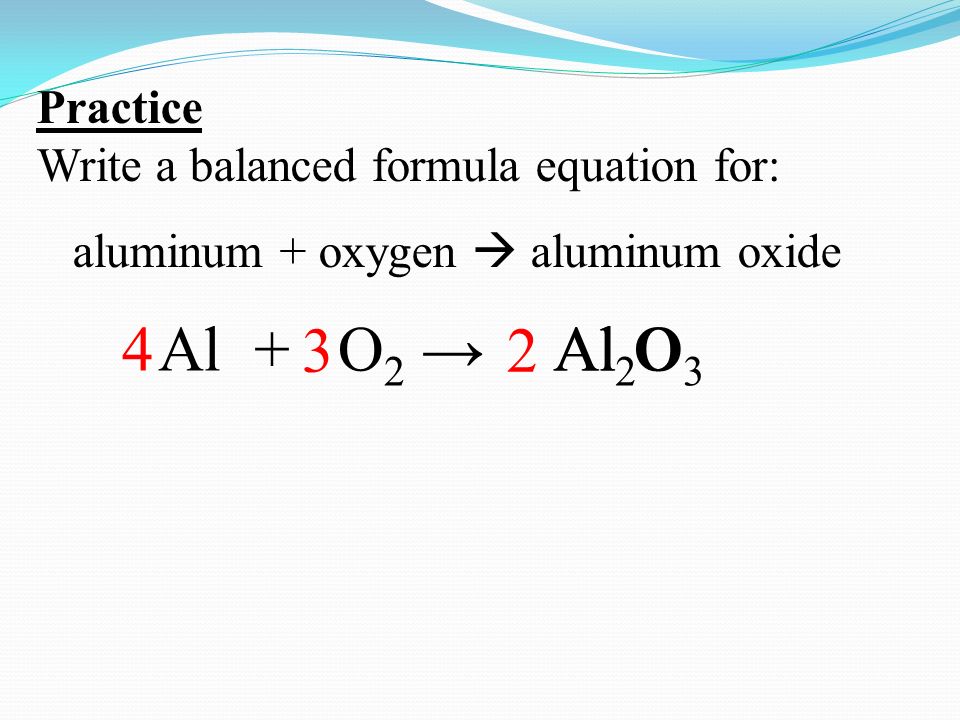
Practice Write A Balanced Formula Equation For Aluminum Oxygen Aluminum Oxide Al Al Oo2 O2 Al 2 O Ppt Download
Comments
Post a Comment